Stereoselektive Silylierung chiraler 1-Aminoallylmetall-Verbindungen mit anschließender Oxidativer Desilylierung
Abstract
Stereoselective Silylation of chiral 1-Aminoallylmetals
and subsequent Oxidative Desilylation
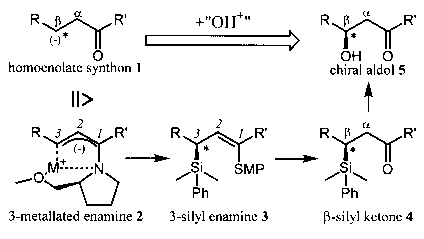
Numerous natural products and pharmaceutical active agents possess a chiral centre in b-position to a carbonyl function. Especially chiral aldol compounds 5 are wide-spread. Besides classical methods for the preparation of chiral aldol compounds 5 the stereoselective introduction of a hydroxyl group into an existing carbon framework represents an essential alternative. By a formal enantioselective linkage of the C-O-bonding starting from a homoeno-late synthon 1 with a "hydroxyl cation" the natural polarity of the reactants is reversed twice. Therefore our aim could be attained only by a temporary introducing of a silyl group. The introduction of a dimethylphenylsilyl group has several decisive advantages: it reacts with 2 regioselectively in 3-position and with high stereoselectivity, it is stable under the strong basic reaction conditions, it facilitates a second metallation by its a-effect, it improves the diastereoselectivity of a subsequent alkylation by its strong steric influence and at last it can be converted into a hydroxyl group with complete retention of configuration.
Metallation of 3-phenyl substituted 1-SMP-aminoallyl compounds with t-BuLi or t-BuLi/KOT leads to chiral 3-metallated enamines 2. These metallated enamines 2 serve as synthesis equivalents of the homoenolate synthons 1 which can be attacked by electrophiles in 3-position. The stereoselectivity is induced by SMP, a Proline derivate, which serves as a chiral auxiliary. Interception reaction of 2 with DMPSCl results in 3-silylated enamines 3. The stereoselectivity of electrophilic substitution can be varied from 64 %ee (S) up to 95 %ee (R) at C3 due to its strong dependence on solvent, metal ion, temperature and size of the chloro silane. The 3-silylated enamines 3 are hydrolysed to b-silyl ketones 4, which serve as precursors for chiral aldols 5.
This strategy could be improved by a one-pot synthesis sequence of metallation, silylation, metallation and alkylation of simple allyl-SMP (R=R'=H) generating almost enantiopure (R)-b-silyl aldehydes. These aldehydes are oxidised and subsequently oxidatively desilylated to b-hydroxy acids or reduced and subsequently oxidatively desilylated to chiral 1,3-diols. Following this concept of silylation and alkylation of allyl-SMP and subsequent oxidative cleavage of the C-Si-bonding, it is possible to prepare a complete library of highly enantiopure chiral aldol compounds.